Register here
By submitting this form, you agree that your data may be stored by the organizer of this webcast. For more info, please contact info@paradigmglobalevents.com
18th Orphan Drugs & Rare Diseases Global Congress 2022 America - East Coast
Attend Orphan Drugs & Rare Diseases Global Congress 2022
18th Orphan Drugs & Rare Diseases Global Congress 2022 Americas will provide a unique platform for the convergence of stakeholders in the orphan drugs industry to discuss and network with top-tier government, hospitals, pharmaceuticals, biopharmaceuticals, non-profit organizations, orphan drugs developers as well as regional and local manufacturers. Network with 120+ representatives from pharma, biotech, patient groups/advocates, government, CROs, and others…
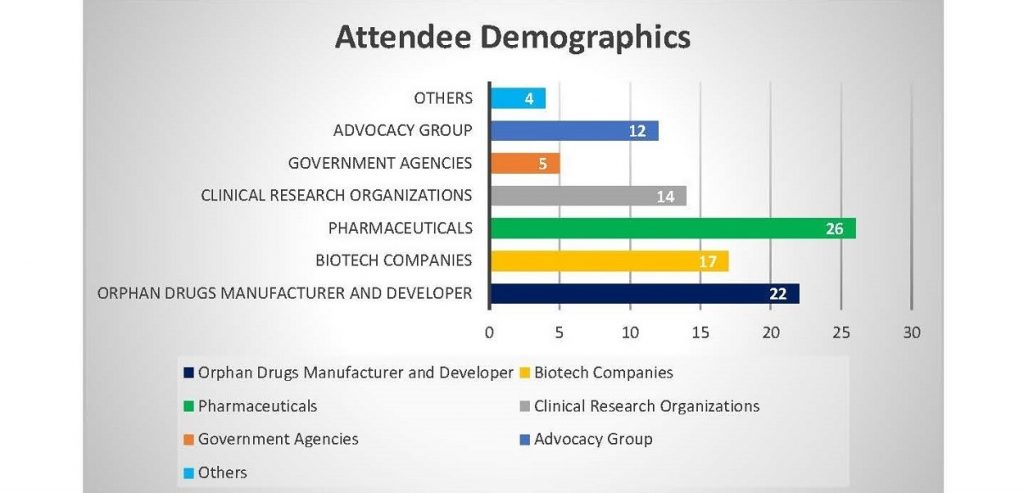
Who Should Attend!
Pharmaceutical & Biotech
- VP Cell,
- Therapy Head of Oncology
- Chief Medical Officer
- Head of Immunotherapy
- Chief Operations Officer
- Heads of Discovery
- VP Clinical Development
- Chief Medical Officer
- Chief Scientific Officer
- Regulatory Affairs
- President, Head of Rare Diseases
- VP Clinical Development
- Chief Medical Officer
- Chief Scientific Officer
- Regulatory Affairs
- President, Head of Rare Diseases
- VP Gene Therapy
- Chief Medical Officer
- Chief Manufacturing Officer
- Chief Scientific Office
- Chief Operating Officer
- Chief Manufacturing &
- Technology Officer
- VP Technical Operations
- VP of CMC
- VP Manufacturing
- VP of Process Development
- VP of Quality Control
- Presidents & Heads of Patient
- Diagnosis
- Programs
- R&D Strategic Alliance
- VP Commercial Operations
- VP Medical Affairs
- VP of Marketing
- VP Medical Affairs
- VP Market Access
- Pricing and Reimbursement
Pharmaceuticals & Biotech
- Chief Commercial Officer
- International Business
- Chief Executive Office
- VP, Head of Market Access
- VP, Head of Pricing and Reimbursement
- VP of Health Economics/HEOR
- VP of HTA
- Head of Value Demonstration
- VP, Evidence, and Data
- Payers (public and private)
- Government & Regulatory agencies
- Chief Data Officer
- Chief Technology Officer
- Chief Operating Officer
- VP, Analytics
- Head of Innovation
- Head of Digital
- MAP Providers
Other Participating Attendees Should Include the following.
- Patient Advocacy Groups
- Rare Disease advocacy organizations
- Newborn Screening
- Hospitals & Healthcare Centers
Payers - Government
- Policy Makers
- Ministry of Health
- Regulators
- Non Profit Organizations
- Academics
- Research Institution
And many more…